GMP System Validation in SAP
Good Manufacturing Practice for pharmaceuticals and active ingredients efficiently implemented in SAP!
The SERKEM Solution in Detail
GMP System Validation in SAP
GMP (Good Manufacturing Practice) refers to a form of ensuring that pharmaceuticals and active ingredients meet the highest quality standards and follow a targeted quality management system. A distinction is made between GMP and cGMP. GMP represents the quality requirements in Germany, while cGMP is the equivalent in the USA.
To comply with GMP requirements, your SAP system must have a comprehensive quality assurance system. Additionally, it should be fully documented and monitored.
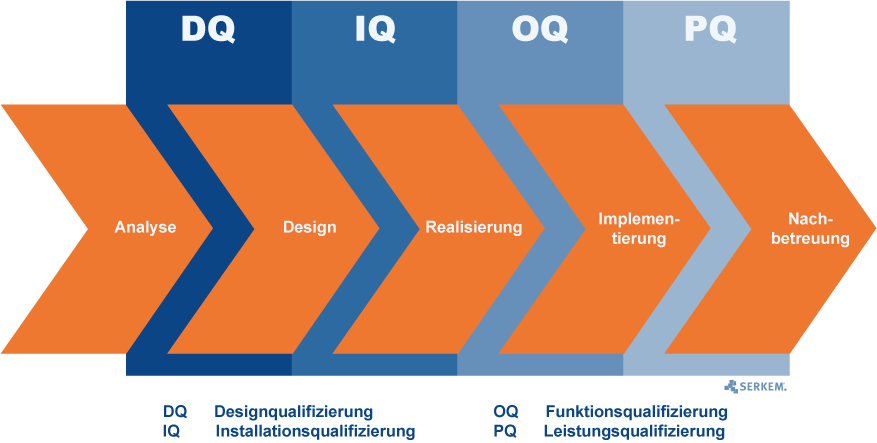
First, a distinction must be made between qualification and validation:
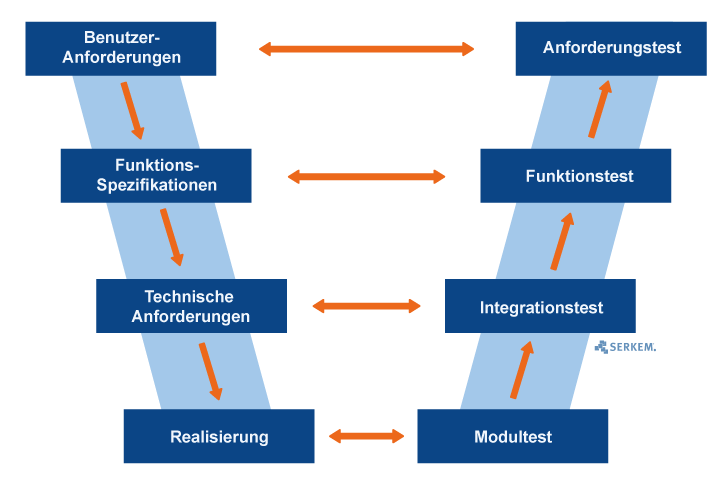
V-model for quality assurance
For the validation of computer-based systems, a so-called V model is often used as reference model. The advantage is that this kind of model can be shown and used in many different forms. Though, this can lead to partially unclear and ambiguous presentations.
The v-model has its name through the characteristically presentation of the letter „v“. The left axis forms the different specification phases and the right axis the relative testing phases.
Therefore, the v-model is very suitable for the presentation of specification phases and the belonging testing phases as wells as for the appropriate adjustment to previously prepared specifications, user requirements and system specifications.
Phases of the v-model
Are these phases completed and the first validation implemented, so the realization follows. Depending on the procedure, the appropriate tests will be executed. So the v-model in particular relation to GMP applies in both implementation steps DQ and IQ.
If you need assistance at the validation of your SAP system, feel free to contact us.